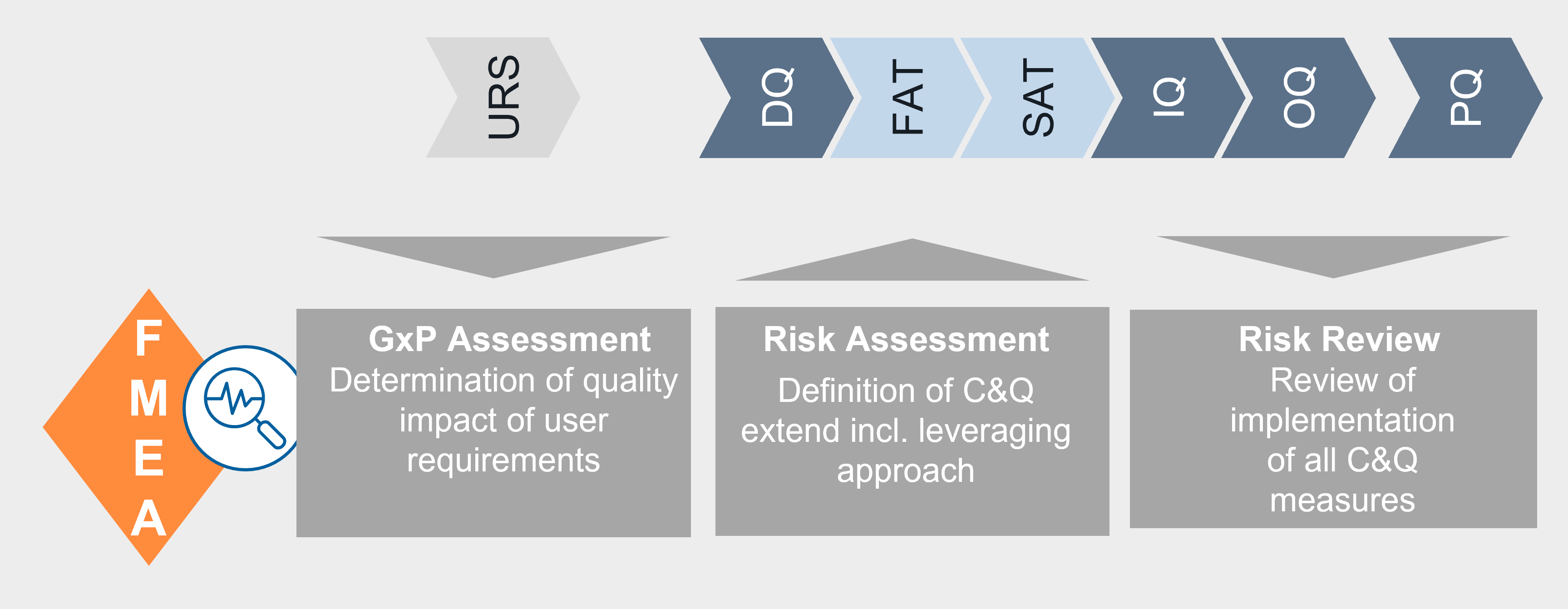
FMEA as the central tool for iC&Q
The FMEA serves as the core element of the risk - based approach - the following phases of iC&Q are mapped here:
• Performance of the GxP-Assessment
• Risk assessment to define the extent of C&Q testing
• Risk Review for tracking of measures
Definition of the C&Q - test scope
The following approach is used to determine whether measures should be implemented during commissioning and/or qualification:
- risk-mitigating measures for GMP-critical requirements can be carried out during commissioning, but their correct implementation must be verified during qualification (leveraging approach).
- risk- mitigating measures for non-GMP-critical requirements (but GEP-relevant requirements) can be carried out only during commissioning - i.e. in the FAT or SAT.
To reflect this approach in the FMEA, each risk-mitigating measure is assigned to a project phase in which it must be performed. The "SAT / IQ (L)" phase - according to the following figure - means, for example, that the implementation of the measure must take place within the framework of the SAT. In the IQ, it must then be checked again whether the measure has been implemented correctly and whether the acceptance criteria have been met. This approach is also known as the leveraging approach.
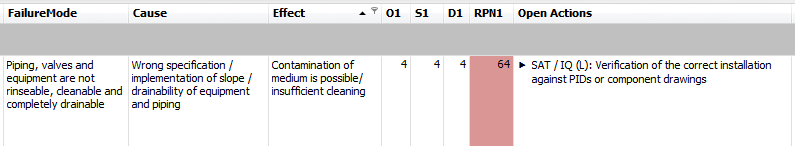
This approach enables a simple and risk-based definition of "what" has to be tested "when" and by "whom" during commissioning and qualification. The "leveraging approach" is used to determine at an early stage of the project what can or must be tested by suppliers from a GMP point of view in the FAT or SAT. This ensures that not only the "standard tests" are carried out during commissioning, but also all tests of the supplier that are necessary for the subsequent qualification by the medical device manufacturer.

![[Translate to English:] [Translate to English:]](/fileadmin/content/REXS/Bilder/header_rexs.png)
![[Translate to English:] [Translate to English:]](/fileadmin/content/REXS/Bilder/integratedCQ.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/content/REXS/Bilder/traceabilitytestmatrix.jpg)